
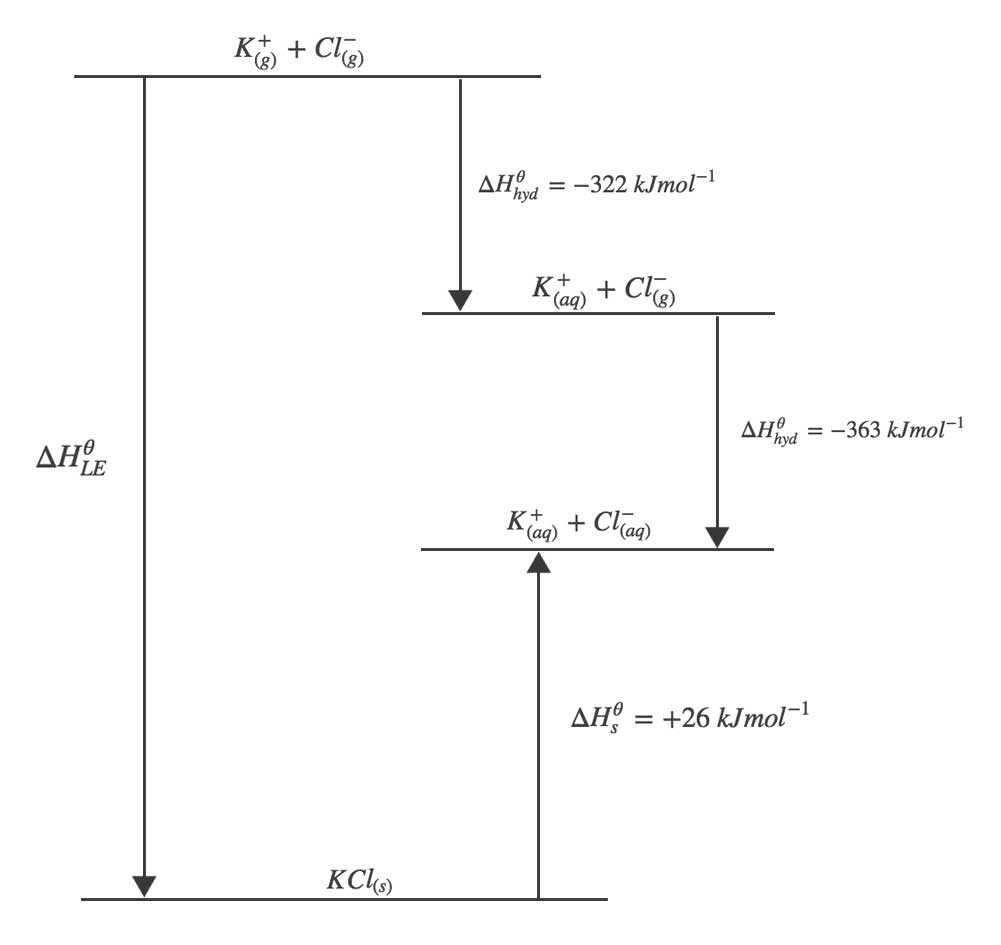
Determine the electron affinities and ionisation energies.Now, discover the dissociation energy and heat of atomisation.First, we have to determine the heat of formation.The measurement is concerned with the lattice energies, and values found from such a procedure are theoretical values.įor finding the lattice enthalpy by using the Born Haber Cycle method, you need to follow the below steps
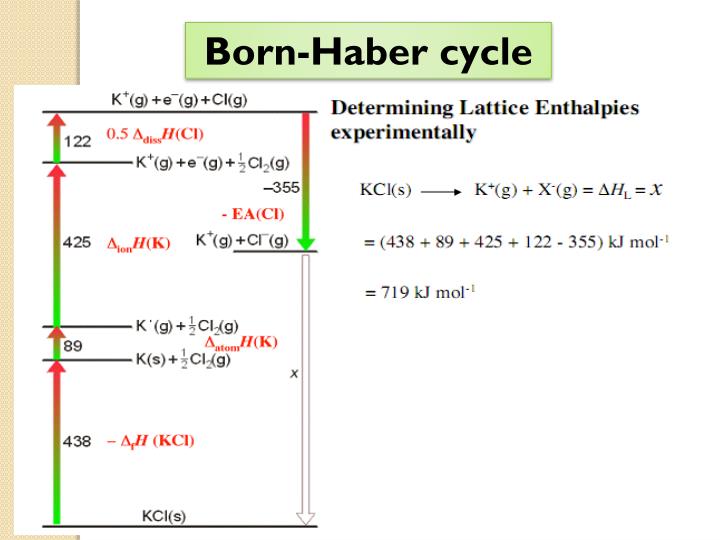
This procedure emphasises how much energy will be released to dissociate the atom from solid to gaseous forms. The lattice enthalpies measured through this method are known to be experimental values. The first way is the born Haber cycle or Hess Law cycle, and the second refers to the physics style.īorn Haber cycle, also known as the Hess Law cycle, is the method that helps calculate the changes in the enthalpy. The lattice enthalpy of the ionic bond can be measured in two methods. However, on the other hand, when energy is needed to create lattice from the gaseous ions, it is termed as the lattice formation enthalpy. NaCl lattice dissociation enthalpies are +787 KJ mol -1. Along with such, we need to understand that the energy needed to separate 1 mole of a solid crystal into gaseous ions is called lattice dissociation enthalpies, and it always remains positive. Lattice enthalpy can be understood in two ways, creation of the solid compounds through the gaseous ions and 2) separating solid into gaseous ions. Therefore, it can be said that the higher the charge magnitude, the higher will be the force.Ī small atom has a small interatomic distance between them, and the inter-atomic distance among them seems to include stronger binding forces, which need higher lattice enthalpy. We need to know that the force available in the lattice crystal seems to be directly proportional to the charge of magnitude. The force is available in the lattice ions, which leads ions to get attracted to each other.

Two important factors influence the lattice enthalpy, i.e., 1) charge of the ion and 2) atom size P = outer pressure FACTORS AFFECTING LATTICE ENTHALPY The Lattice enthalpy formula is written as
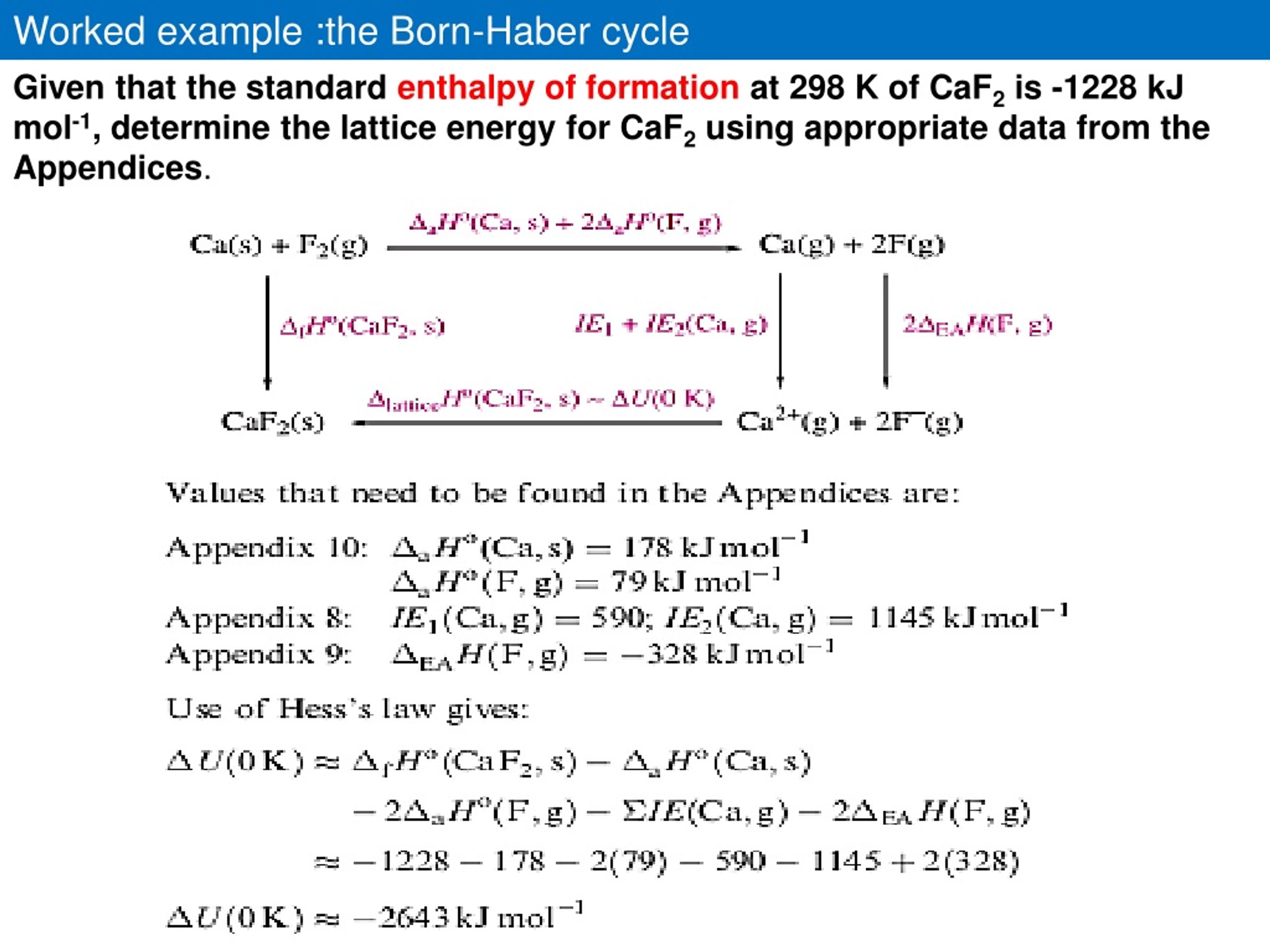
The lattice enthalpy is defined as the measure of the strength of the ionic compound. This article will discuss the method of calculating lattice enthalpy and include some examples for better understanding. The energy required to separate one mole of the solid ionic compound into the constituent gaseous ions is lattice enthalpy. The molecules present in the ionic solids have been arranged in the 3-D grid-like structure known as the lattice structure. The ionic compounds are usually present in the solid form, and their molecular force seems very strong.


 0 kommentar(er)
0 kommentar(er)
